Xebios Diagnostics Group

News
Legionella Analytics: New Comparison Study of University of Turin:*
Sensitivity and Selectivity of Two Commercially Available Media for Legionella spp.
Recovery from Environmental Water Samples – Xebios culture media best in class!
COLIKAT RAPID:
Now available: COLIKAT RAPID, Enumeration of Escherichia coliand coliform bacteria in 18 hours.
COLIKAT RAPID Website Our products Contact
*Publication:
Ditommaso, S.; Giacomuzzi, M.; Memoli, G.; Garlasco, J.; Zotti, C.M. Sensitivity and Selectivity of Two Commercially Available Media for Legionella spp. Recovery from Environmental Water Samples. Pathogens 2020, 9, 523.
About us
Xebios manufactures high-quality ready to use culture media for microbiological diagnostics. We are dedicated to be your system partner in securing the quality of your laboratory services.
As your system partner we do not only reliably provide you with your ready to use culture media, we also offer support for your internal quality control system and help you to sustainably reduce your cost for quality control. We make sure that all necessary materials are provided at all times to secure smooth processes at your laboratory.We have 25 years of experience with diagnostics work in the microbiological laboratory and therefore know the requirements and daily challenges of our customers by heart. We understand your needs and know how important a good supplier for diagnostics culture media is for your work. This is why we can provide you more comfort supplying your ready to use culture media and contribute to your success.
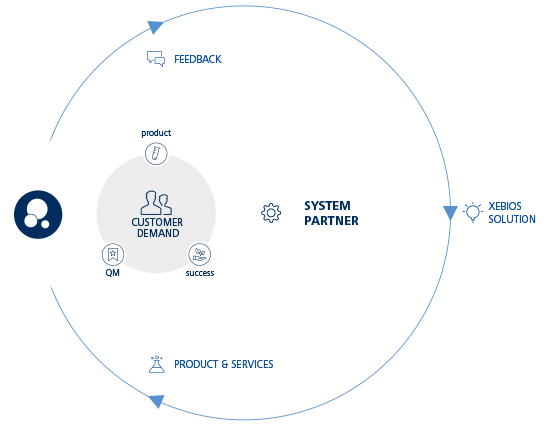
Service outline
Your benefit
Quality partnership for ISO 11133
We support you with all questions concerning quality control for your ready-to-use culture media and offer a 360°-solution for ISO 11133 in your laboratory:- Xebios is certified according to ISO 9001. We comply with all requirements of ISO 11133 for manufacturing, storing, quality control and performance testing of ready to use culture media.
- The microbiological quality testing of our ready to use culture media is carried out by our laboratory that is accredited according to ISO/IEC 17025 for testing of diagnostic culture media according to ISO 11133.
- We test the membrane filter that you are currently using in your laboratory in combination with our culture media – You receive an individual combination certificate according to ISO 11133 sec. 7.3 and ISO 7704 containing your membrane filter’s and our culture media’s batch number.
- We have our own supply logistics in house and we will deliver your products in our own refrigerated trucks. As we control the whole supply-chain, we are able to consistently document the storage and temperature conditions during the transport. You will receive a quality certificate which confirms that all storage conditions were kept so that no additional transport validation is required (ISO 11133 sec. 6.4.2).

Service comfort
As your system partner we are dedicated to offer the best service comfort for the daily routine in your laboratory:- Our Xebios-App enables you to monitor and manage your stock, consumption and demand for ready to use diagnostic culture media. You have an easy way to keep the overview about your inventory levels and will be reminded in time if critical materials run short. I you want the system will suggest new orders with optimal amounts at the optimal time.
- We offer the highest flexibility for individual products and service solutions, also in small batch sizes – Please get into contact with us: We are pleased to develop customized solutions for your laboratory in cooperation with you.
- If required, our laboratory will perform the testing of our ready to use media in cooperation with your staff and according to your internal methods.
- We visit you periodically with our own trucks and will help you to carry your delivery directly to your warehouse if required.
- We use reusable boxes. This simplifies the handling of your ready to use media in the laboratory and significantly reduces packaging material and waste.

Cost reduction
As your system partner, we actively help you to reduce your costs:- We comply with all requirements of ISO 11133, ISO 13485 and Ph. Eur. to the manufacturing, quality control and performance testing of our ready to use diagnostic culture media. You benefit, as work-intensive quality testing of incoming media is no longer necessary, remaining at a constant quality-level.
- We offer individual reservations for whole batches according to your requirements and to your demands. Your cost for quality controls will be reduced because a new delivery of media does not necessarily lead to a new batch.
- We consistently control the storing conditions of our ready to use media from the manufacturing until the delivery to your laboratory. You save additional transport validation and documentation.
- We test the combinations of your ready to use media and the membrane filters that are used in your laboraroty according to ISO 11133 and ISO 7704. You will receive individual quality control certificates. You receive a product which is individually tested and certified for you and that is based on your individual needs.
- Our Xebios-App guarantees a clear presentation of your inventory, consumptions, stock and demands for your diagnostic culture media. We archive and adminstrate all quality certificates (product, filter, transport) for you, to significantly reduce paper work in your laboratory.
The easiest way to the right product:
The product you are looking for is not listed?
Please contact us at any time. We are expanding our product portfolio continiously and would be happy to inform you about the latest news. Furthermore we can make your individual product to your demands on request. Our service team will be happy to help you with all questions.
Quality commitment
At Xebios we personally guarantee for the quality and the reliability of our products and services.
Our procedures to manufacture, store and the performance of the quality control of our products comply with all requirements of ISO 11133 and follow the "Good Manfacturing Practice (GMP)". We measure our success by meeting the high-quality demands of our customers at all times.
Our suppliers for our raw-and input materials, technical equipment and machinery are reliable and established partners with long-proven quality.
Jobs
The Xebios Diagnostics Group is growing. Heute beschäftigen wir rund 90 Mitarbeiter an drei Produktionsstandorten in Düsseldorf, Eindhoven (NL) und Groningen (NL). In order to further expand our services and offers, we are constantly looking for new employees (m/f/d) and we look forward to receiving your application.
Contact
We are available at all times to answer your questions about our products or services and to prepare an individual offer for you.
Please contact us using the contact data mentioned below or via our contact form. 
Xebios Diagnostics Group GmbH
Telleringstraße 49
40597 Düsseldorf
T : +49 211 78 88 07 - 00
E : info@xebios.com
Contact/product request
Please note:
General Terms and Conditions
General Terms and Conditions of Purchase